| Composition: |
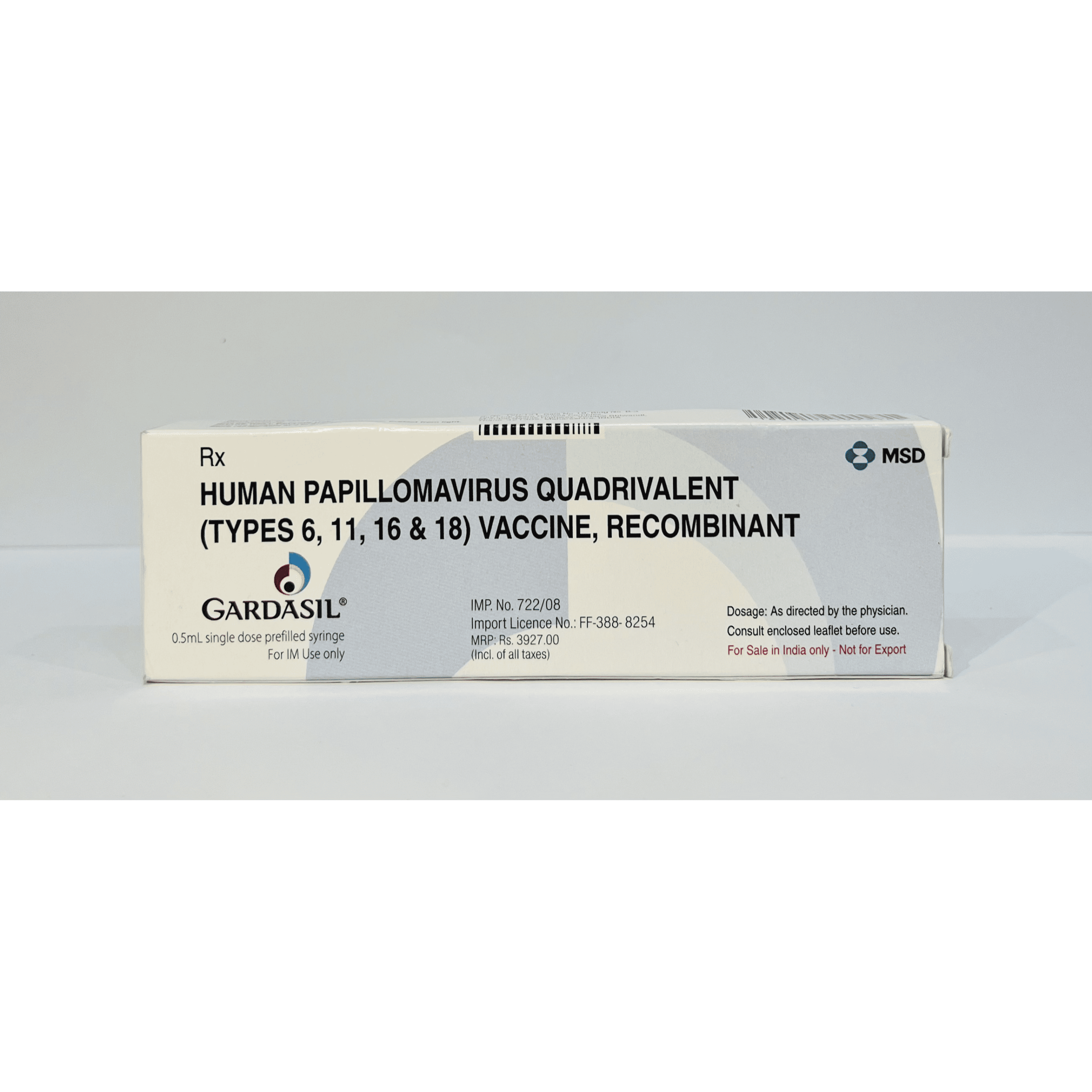
Human Papillomavirus Quadrivalent Vaccine
(Type 6,11,26 & 18) HPV |
|
|
Description:
Gardasil is a vaccine designed to protect against infections caused by four types of Human Papillomavirus (HPV) – types 6, 11, 16, and 18. HPV types 16 and 18 are responsible for approximately 70% of cervical cancer cases, while types 6 and 11 cause about 90% of genital warts. Gardasil helps prevent cervical, vulvar, vaginal, and anal cancers, as well as genital warts in both males and females.
Gardasil stimulates the immune system to produce antibodies against HPV types 6, 11, 16, and 18, offering protection before exposure to the virus. It does not treat existing HPV infections or associated diseases but helps prevent new infections.